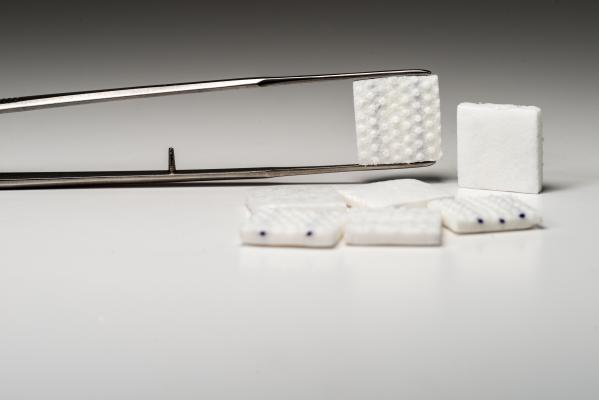
Through a novel self-irrigating mechanism, Thoraguard eliminates one of the main hazards of analog chest tubes: the need for medical personnel to keep a constant eye out for blockages and other irregularities.
Centese Develops Game-Changing Device for Surgery Patients