Deep Blue is a Rosenman Innovator company.
From the press release.
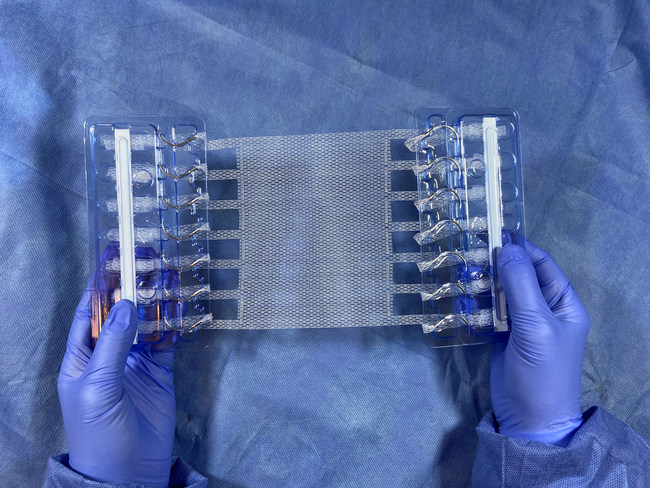
Deep Blue Medical Advances today announced it has received 510(k) Clearance from the U.S. Food and Drug Administration (FDA) for its T-Line® Hernia Mesh with integrated suture-like extensions. T-Line Hernia Mesh provides superior anchoring strength and eliminates a key point of failure for conventional mesh fixation – the mesh, suture, tissue interface that often leads to mesh migration, contraction and eventual failure.
Hernia occurrence and recurrence is often a problem following abdominal surgery, when abdominal pressure (e.g. from lifting or coughing) can cause sutures to cut or pull through the tissue or mesh. Invented by surgeons, Deep Blue’s large-pore, mid-weight T-Line Hernia Mesh is designed to increase anchoring strength and prevent mesh fixation failure. The mesh extensions have 15x more surface area than traditional sutures and act similar to how snowshoes prevent sinking into snow, reducing fixation stress by spreading force over a greater area. T-Line mesh extensions are designed to withstand significant abdominal pressures (in literature, up to 50 N/cm of stress), which is beneficial for all patients, including Grade II patients with comorbidities.
“Sewing a bit of each extension into the abdominal wall, in lieu of traditional sutures, significantly increases mesh anchoring strength and thus the durability of the repair,” said Dr. Howard Levinson, Deep Blue’s founder. “We believe this approach will greatly improve patient outcomes without necessitating significant changes to current surgical practice.”
“In literature, long term hernia repair failure rates are 32% using conventional mesh and 63%1 using suture alone, creating a multibillion-dollar clinical cost to the US healthcare system. Deep Blue’s products enhance hernia surgery with a potentially significant impact,” said CEO Bill Perry. “Furthermore, extensive lab and benchtop testing indicate that the T-Line Mesh has ~275% greater anchoring strength than standard of care in the perioperative period. This is always important, but particularly so in the period before significant tissue in-growth into the mesh has occurred.”
The company expects to clinically launch the product in selected sites in Q3, 2020.
About Deep Blue Medical Advances
Established in 2015, Deep Blue Medical Advances is dedicated to addressing the unacceptably high rate of hernia occurrence and recurrence. It manufactures the T-Line® Hernia Mesh.