iSono is a Rosenman Founders Pledge company. From the press release
iSono Health, Inc. today announced U.S. Food and Drug Administration (FDA) clearance of the company’s ATUSA™ System an innovation in medtech for breast imaging, a first-of-its-kind compact automated whole breast ultrasound system featuring a unique wearable accessory and an intuitive software for automated image acquisition and analysis.
“Breast cancer is the leading cause of cancer death among women worldwide, and one in eight women will be diagnosed with breast cancer in their lifetime. We founded iSono Health with the vision to enable earlier diagnosis and treatment for breast cancer to save women’s lives, and this FDA clearance is a major step to fulfilling that vision,” said Maryam Ziaei, Ph.D., co-founder and CEO of iSono Health.
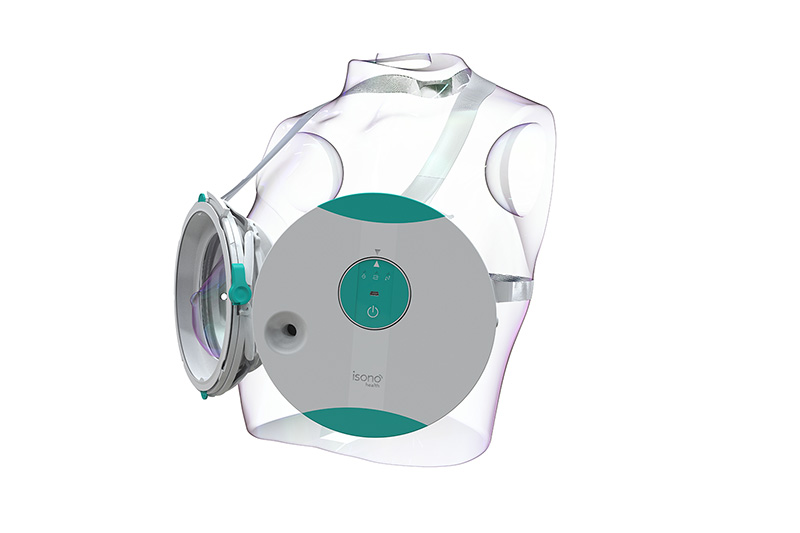
Designed to offer enhanced efficiency, consistent accuracy and a comfortable patient experience, ATUSA’s patented technology makes 3D breast ultrasound imaging accessible to patients and physicians at point of care. In just two minutes, the portable ATUSA system automatically scans the entire breast volume, independent of operator expertise, and offers 3D visualization of the breast tissue. The ATUSA system is designed from the ground up to seamlessly integrate with advanced machine learning models that will give physicians a comprehensive set of tools for decision making and patient management.
“A portable and automated whole breast ultrasound augmented with machine learning would be the most practical technology to reduce breast cancer mortality globally, specifically in countries with limited resources. In developed countries, ultrasound has been proven effective as an adjunct to mammography to improve breast cancer diagnosis in women with dense breasts,” said Mohammad Eghtedari, M.D., Ph.D., a breast radiologist and an assistant professor at University of California San Diego. “I believe iSono Health’s automated and portable technology will improve access to breast care for women worldwide and empower physicians with insightful data to make more informed clinical decisions.”
This is the first of several intended FDA submissions for the company. Currently, iSono Health is conducting prospective case collection studies to further validate various deep learning models integrated with ATUSA software to aid clinicians in localization and classification of breast lesions.
“Clinicians and women worldwide need high quality breast imaging that is accessible and efficient at scale, without the need for highly skilled operators,” adds Ziaei. “The portable and automated ATUSA system stands alone in comparison to other ultrasound offerings in promising to address that need. We are thrilled to be able to get ATUSA into the market and have the opportunity to help save lives.”
iSono Health is supported by a National Science Foundation SBIR grant, Y Combinator, Foothill Ventures, Draper Associates, Plexo Capital, ATO Ventures, Camford Capital, as well as JLABs, Texas Medical Center Innovation and other top medical centers.
About iSono Health
iSono Health was co-founded by two female engineers with PhDs who shared similar personal experiences with the inadequacies of mammograms, especially for women with dense breasts, that sparked the passion to find a better way for breast health monitoring. iSono Health’s mission is to make personalized whole breast imaging accessible to all women worldwide. iSono Health has an experienced team with extensive experience in medical imaging and AI. For more information visit www.isonohealth.com or find us on Twitter, Facebook and LinkedIn.
iSono Health, ATUSA, atusa are trademarks and/or registered trademarks of iSono Health, Inc., and/or its subsidiaries in the United States and/or other countries.
1 U.S. Food & Drug Administration 510(k) Premarket Notification. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K213620