Velano is a Rosenman Founders Pledge member. From MassDevice.
BD (NYSE:BDX) earlier this year announced a move that it believes can change the standard of care in blood collection.
With the acquisition of Velano Vascular and its needle-free technology for high-quality blood draws, the company aims to transform patient experience, according to Rick Byrd, BD’s worldwide president of medication delivery solutions.
“Needle sticks can be one of the most traumatic aspects of a hospital stay,” Byrd told MassDevice in an interview. “For the patient, obviously, but they’re also anxiety-ridden for the phlebotomist and the nurse who has to perform the needlesticks. … The acquisition of Velano Vascular adds an innovative, needle-free blood draw technology to our portfolio that will reduce the pain and discomfort of multiple needle sticks.
“We believe it will create a whole new standard of care for blood collection.”
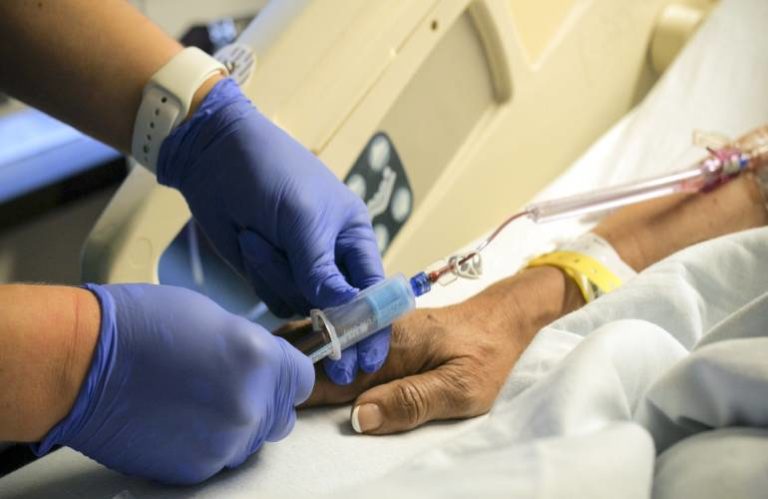
The acquisition — of which terms were not disclosed — represents the platform on which BD is building its “One-Stick Hospital Stay” blood collection solution, an initiative that aims to reduce the usual 10-20 needle sticks per hospital stay in those who require blood draws, according to Byrd, to just one stick.
Velano’s FDA-cleared PIVO device attaches to existing peripheral intravenous catheter (PIVC) lines, paving the way for the One-Stick blood collection and vascular access initiative by avoiding blood draws performed through a puncture separate to the IV catheter.
“Velano is very much focused on this idea of delivering painless, compassionate care for inpatient hospitalization and providing a safer practice as well,” Byrd said. “Their ambitions were very much aligned to BD. We’re super excited to join forces with them.”
San Francisco–based Velano Vascular was named the fastest-growing medical device company in 2020 by Deloitte and, according to Byrd, already has a strong customer base with primary healthcare facilities using its technology.
Its system holds FDA clearance and BD’s plans to go beyond making it the standard of care in the U.S. include registering the platform across the world to globalize Velano’s offerings.
“They have a very good base of customers and this is very synergistic to our BD products, to our commercial models and our sales teams,” Byrd said. “We’ve immediately hit the ground running with this.”
Byrd said BD will take the necessary steps to ensure that it continues to innovate upon Velano’s technology, making the high-quality specimen management platform compatible with all BD catheters, then compatible with all IV catheters.
The aim is to leverage its expertise to try and draw as much utility out of the already-placed IV catheter as possible, demonstrating the catheter’s capabilities beyond delivering medication.
“With BD, we’re committed to transforming vascular care and driving innovation to improve clinical outcomes and better serve our patients,” Byrd said. “We were really excited to announce this big step forward that we took in our mission to transform the patient experience through this vision.”