Ajay Dharia, MD, is Principal at our investment firm MedTech Venture Partners, and a practicing pulmonary and critical care doctor at Bay Area hospitals including UCSF Medical Center. He is an advisor to the CPAP/BiPAP project at Berkeley.
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
From UC Berkeley Bioengineering. By Sarah Yang
With some simple modifications, consumer devices used to treat sleep apnea could be converted into life-saving ventilators for patients with COVID-19, according to a coalition that includes UC Berkeley engineers, emergency room doctors and critical care pulmonologists. The solution could help alleviate the critical shortage of ventilators as the number of COVID-19 patients surges and hospitals scramble for needed supplies.
“Tens of thousands of COVID-19 patients in this country and around the world will need respiratory support in the coming weeks and months,” said Grace O’Connell, the Don M. Cunningham Endowed Professor at UC Berkeley’s Department of Mechanical Engineering and a leading member of the coalition. “We believe that using sleep apnea machines is a viable solution for non-ICU patients. This way, higher-grade ventilators can be reserved for patients with more advanced stages of respiratory disease.”
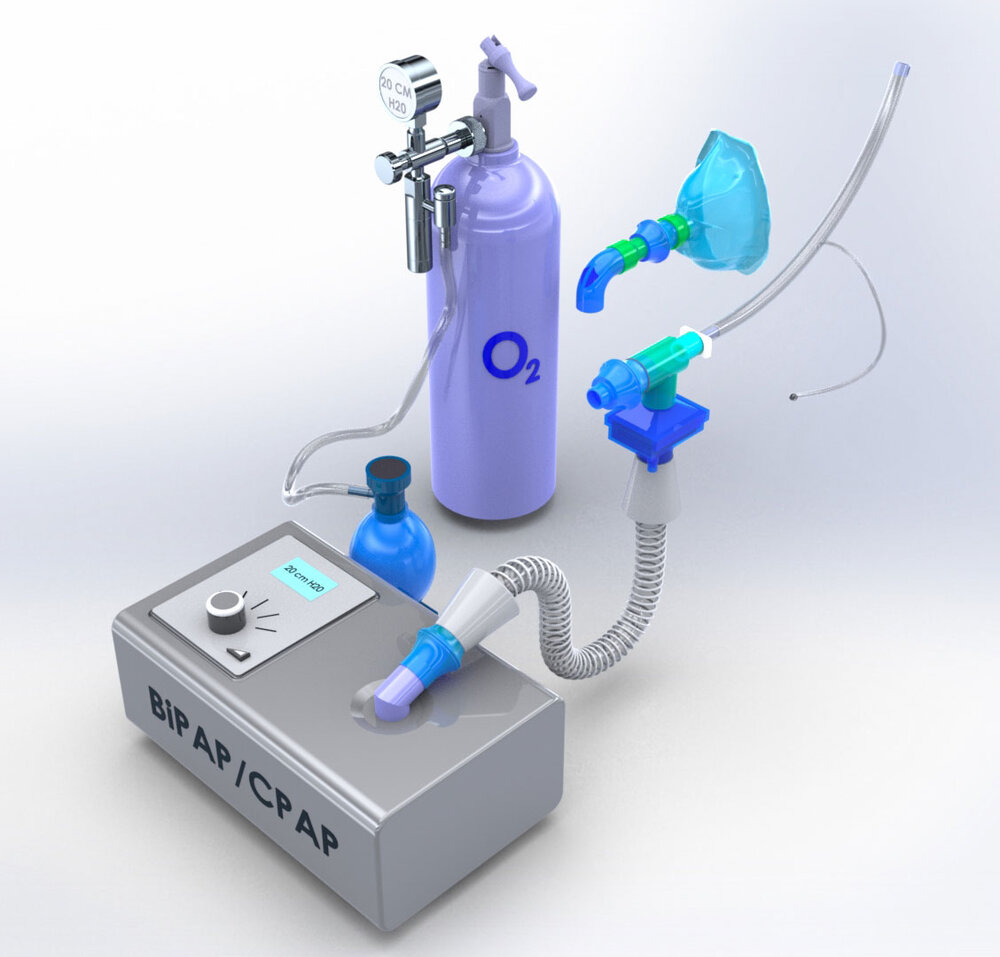
The U.S. Food and Drug Administration recently issued guidelines that allow for continuous positive airway pressure (CPAP), auto-CPAP, and bilevel positive airway pressure (BiPAP or BPAP) machines, typically used for treatment of sleep apnea, to be used to support COVID-19 patients experiencing breathing problems as long as the patient is appropriately monitored.
The new modifications allow the CPAP sleep apnea machine to accept oxygen where ambient air enters the device. The oxygenated air is then filtered and delivered to a patient through an FDA-approved endotracheal tube. Lastly, the exhaled air is re-filtered before being released into the surrounding environment.
By using an endotracheal tube to deliver oxygenated air through a two-filter system, the machine bypasses the need for face masks, which could spread disease if aerosolized viruses escape.
O’Connell points out that these machines are not meant for the most serious cases of COVID-19, where patients are in need of higher-pressure air from medical grade ventilators.
“Our solution could be used for those patients who need support for mild to moderate respiratory symptoms, saving the ventilators for ICU patients who are experiencing acute respiratory distress,” said O’Connell.
Companies and medical organizations have looked into the use of medical-grade BiPAP machines as ventilators, but the benefit of using consumer sleep apnea machines is that they are less expensive, far more prevalent and do not have long lead times on manufacturing, according to coalition members
The American Sleep Apnea Association estimates that 22 million Americans suffer from sleep apnea, and industry estimates of CPAP use range from 8 million to 10 million in the United States. Many sit unused because they are uncomfortable to use on a daily basis, the coalition members said.
“A lot of people don’t need or use them,” O’Connell said. “So how can we repurpose these devices to be used as ventilators in hospitals during an emergency?”
Using an off-the-shelf CPAP sleep apnea machine, O’Connell and her team of UC Berkeley students designed the ventilator with the help of Berkeley Engineering alumni Bryan Martel and Ajay Dharia, MD, and Bert Lubin, MD, former president and CEO of the UCSF Benioff Children’s Hospital Oakland and now a special advisor on health at Berkeley Engineering and the Blum Center.
The UC Berkeley engineers are continuing to explore additional modifications with parts produced by 3D printers at the Jacobs Institute for Design Innovation and the CITRIS Invention Lab. UC Berkeley officials have allowed both maker spaces to continue operating to support research related to COVID-19.
Because these sleep apnea machines have already been approved by the FDA to help increase oxygen flow, and the parts used to modify the machine are FDA-approved, the researchers say the modified machines could potentially be deployed quickly.
“People are dying. There is a real urgency to this effort,” said Martel.
Members of the coalition are currently reaching out to local and state government officials, calling for a partnership among doctors, the FDA, manufacturers and logistic companies. They have also set up a website, VentilatorSOS.com, where members of the public can donate their unwanted sleep apnea machines.
Seed funding from the Berkeley Engineering Fund provided support for this project.