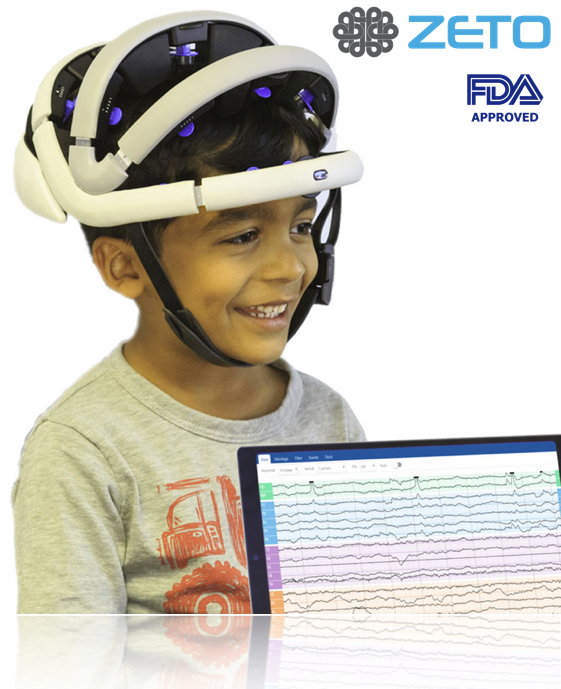
Congratulations to Zeto and its CEO/Founder Aswin Gunasekar. The FDA has recently approved their product for clinical use. Zeto’s “dry electrode” headset, is the first of its kind to obtain FDA approval, enables doctors to obtain EEGs quickly and conveniently to 10-20 EEG specifications. The easy-use cloud platform makes EEG available to any health care facility.